Discovering Lyme
Many believe that the bacterial infection now called “Lyme disease” has been causing illness among humans for thousands of years. Cases were first documented in Europe in the 1880s, but as recently as September 1991, scientists discovered the presence of the Lyme-causing bacteria in a frozen 5,300 year-old mummy found in the Austrian Alps. An autopsy of the mummy conducted by scientists in November 2010, and a DNA analysis conducted the following June, revealed the genetic footprint of Borrelia burgdorferi —making the Iceman called “Oetzi” the earliest known human infected by the bacteria that causes Lyme disease (Hall 2011).
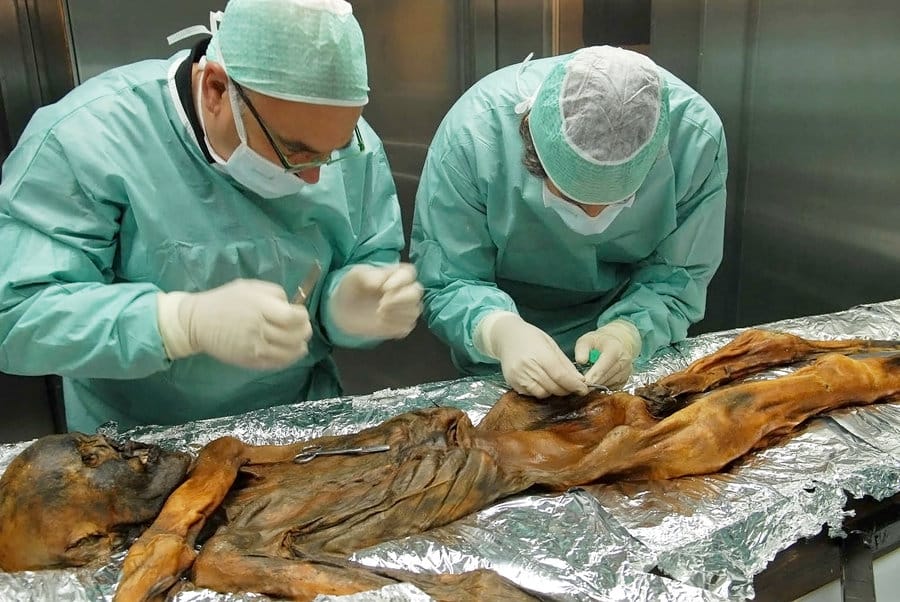
After “Oetzi,” there have been many thousands, perhaps millions, of Lyme sufferers. Lyme disease is currently the fastest-spreading vector-borne disease in the United States. According to the U.S. Centers for Disease Control and Prevention (CDC), as many as 300,000 or more new infections likely occur annually.
CDC statistics from 2001 to 2010 place boys between the ages of 5 and 9 at the highest risk for Lyme infection due to more time spent outside or playing with pets. Indeed, children were integral to the identification of Lyme disease as a new, tick-borne bacterial threat during the summer of 1975 in the coastal town of Lyme, Connecticut. A concerned mother noticed an unusual cluster of juvenile rheumatoid arthritis cases and asked Yale University to conduct an investigation. Investigators concluded that an infection was responsible for both the arthritis and the neurological symptoms, such as Bell’s palsy (paralysis of half the facial muscles) and heart rhythm abnormalities, which were emerging among the patients. Many of the affected children reported finding a circular red rash with a central clearing that resembled a target or “bull’s-eye” prior to the onset of their other symptoms. This clue helped investigators realize that the bacteria were probably spreading through the bite of an infected tick, spider, or insect. Eventually, the small deer tick (Ixodes scapularis species) was identified as the culprit.
Finding the Cause
Researchers soon discovered the cause of Lyme symptoms to be a spiral-shaped bacteria called a spirochete. This same class of bacteria also causes syphilis and periodontal disease as well as other ailments (Burgdorfer et al., 1982). The species responsible for Lyme symptoms was named Borrelia burgdorferi after its discoverer, Dr. Willy Burgdorfer of the National Institutes of Health (NIH).
The experts also attributed a plethora of signs and symptoms to Lyme disease. Its manifestations were found to be so broad that it earned the nickname “the great imitator” of dozens of other medical conditions (Pachner, 1988; Blanc & Gebly, 2007). As researchers at a Philadelphia hospital noted in 2000, “Lyme neuroborreliosis is diagnostically challenging because of its diverse manifestations” (Zhang et al., 2000). Frequent symptoms include incapacitating fatigue (Steere et al.,1984), musculoskeletal “joint” pain (Steere et al., 1983, Schmid, 1989, Steere et al., 1988), pain and odd nerve sensations in the extremities (Halperin et al., 1987; Shadick et al., 1994; Weissenbacher et al., 2005; Fallon et al., 1998), and cognitive and mood disorders such as memory loss (Logigian et al., 1997; Krupp et al., 1991; Kaplan et al., 1992), concentration difficulties (Reik et al., 1995, Sigal, 1990; Vrethem et al., 2002) and depression (Logigian et al., 1997; Duray & Steere, 1988.
Impact on Society
Studies have shown that the financial and emotional cost of disseminated Lyme disease can be severe in addition to the many physical afflictions. “Persons with a history of Lyme disease have more musculoskeletal impairment and a higher prevalence of verbal memory impairment when compared with those without a history of Lyme disease. Our findings suggest that disseminated Lyme disease may be associated with long-term morbidity.” (Shadick et al., 1994). Seven years later, researchers announced that patients with chronic symptoms following a bout with Lyme disease suffer a quality of life equal to someone with osteoarthritis or congestive heart failure and worse than someone with Type 2 diabetes or a recent heart attack (Klempner et al. 2001). Finally, March 2008 witnessed the publication of a Columbia University study which reinforced these earlier findings, and added that patients complaining of chronic Lyme disease also suffer fatigue comparable to that caused by multiple sclerosis (Fallon et al., 2008).
Controversy
In addition to the debate over the manifestation of disseminated or chronic Lyme disease, serious dilemmas in the diagnosis and treatment of Lyme disease remain unresolved. Johns Hopkins University announced that a clinical diagnosis of Lyme disease – one based on the patient’s history and symptoms rather than on blood or urine tests – is “still the most appropriate approach,” because “the degree of sensitivity needed for a high level of assurance at the time of early Lyme disease is still not obtainable, even through combinations of various laboratory tests” (Coulter et al. 2005). Current Lyme serological testing is known to lack both sensitivity to the presence of the infection, and specificity, which is the ability to distinguish between Lyme and similar spirochetal infections, such as syphilis and periodontal disease. Unreliable testing can result in false positives and more often, false negative results. This underlies Johns Hopkins’ recommendation to rely on patient history and complaints rather than currently available blood tests for a more accurate diagnosis.
Perhaps the most contentious area of debate revolves around patients who have received a month or more of antibiotics for Lyme disease yet continue to suffer from persistent symptoms. The Infectious Disease Society of America (IDSA) acknowledges in its 2006 treatment guidelines for Lyme and two other tick-borne infections that “unexplained chronic subjective symptoms” persist in “a minority” of patients who have already received “treatment with conventional courses of antibiotics for Lyme disease” (Wormser et al., 2006). Although not offering a firm or thorough explanation for this phenomenon, the IDSA suggests that these symptoms may result from an undiagnosed and untreated tick-borne co-infection—which would produce symptoms similar to Lyme—or even an emotional reaction to the past infection with Lyme. The IDSA authors also note; “In many patients, post treatment symptoms appear to be more related to the aches and pains of daily living rather than to either Lyme disease or a tick-borne co-infection. Put simply, there is a relatively high frequency of the same kinds of symptoms in ‘healthy’ people” (Wormser et al., 2006). Thus, Wormser and his co-authors dismiss some patients’ complaints of fatigue, pain, and memory dysfunction due to “the high frequency of identical complaints in the general population” (2006). Accordingly, the IDSA recommends that doctors seek alternative diagnoses for these complaints and provide therapies for symptom relief.
Research has suggested that “post-Lyme syndrome” (as the IDSA prefers to call it) results from an autoimmune reaction to the Lyme bacteria, in which the immune system does not shut down after the Lyme bacteria are eliminated, resulting in damage to healthy, normal body tissues. Studies have shown that some people possess certain genetic traits called HLA-DR4 and HLA-DR2, which result in a severe, persistent, and autoimmune-like arthritic reaction when they are exposed to the Lyme spirochete, whether in a vaccine or in nature (Steere et al., 1990). This theory might explain the depth of suffering that some people experience once infected with Lyme and the ability of Lyme to mimic autoimmune conditions. A significant effort to identify a common genetic marker besides HLA-DR4 and HLA-DR2 among all those claiming to suffer “post-Lyme” symptoms failed (Klempner et al., 2005), leading some to abandon this theory as insufficient to explain “post-Lyme” suffering.
A third explanation for continuing Lyme symptoms, embraced by many physicians and patients, is that the Lyme bacterium is capable of surviving the short-term doses of antibiotics currently administered, making it a persistent infection. A significant number of studies have documented the cultivation of live Lyme spirochetes or their DNA from the bodies of patients who have already received one or more rounds of antibiotics (e.g., Oksi et al., 1999; Breier et al., 2001; Hudson et al., 1998). Some studies, written by prominent members of the IDSA, have posited persistent Lyme infections as the cause of persisting symptoms (Halperin and Heyes,1992; Liegner et al., 1993; Dattwyler et al., 1988; Breier et al., 2001; Steere et al., 1988; Logigian et al., 1990). Still other studies have explored the exact mechanisms by which the spirochetes are believed to survive either antibiotics or the immune system (Klempner et al., 1993; Georgilis et al., 1992; Liang et al., 2002; Diterich et al., 2003; Seiler and Weis, 1996; Murgia et al., 2002; Duray et al., 2005; Brorson and Brorson, 1998; Alban et al., 2000; Barbour et al., 1982; Radolf et al., 1994; Benach, 1999; Barbour and Hayes, 1986; Sadziene et al., 1994; Kurtti et al., 1987; Aberer et al., 1996; Mursic et al., 1996; Embers et al., 2004; Garon et al., 1989; Livengood & Gilmore, 2006; Ma et al., 1991; Dorward et al., 1997; Jackson et al., 2007.
Treatment Strategies
Clinicians who believe that Lyme can be a persistent and relapsing infection often treat their patients with combinations of antibiotics over a long period until symptoms resolve or subside. This is not in accord with the short-term treatment schedules set forth by the IDSA. This alternative approach to treatment has put these physicians, frequently called “Lyme-literate” doctors, at odds with the IDSA, which denies that the Lyme bacteria persists in the human body after a month or two of antibiotic therapy.
Many medical professionals who espouse long-term treatment of a resilient Lyme infection organized themselves into a medical society in 2003 called the International Lyme and Associated Diseases Society (ILADS). They began a dialogue with the IDSA and other sectors of the medical community about the growing threat of Lyme and tick-borne infections, which do not seem to resolve in some patients. Unfortunately, the emotional and heated debate over the possibility of persistent Lyme infection and the best way to provide relief to suffering patients continues. ILADS doctors who disagree with the short treatment recommendations of the IDSA sometimes find themselves investigated and tried by their state medical licensing board for breaking with the IDSA protocol. IDSA’s 2006 Lyme treatment guidelines, quoted above, faced an antitrust, monopolization, and exclusionary conduct investigation by the Attorney General of Connecticut, Richard Blumenthal. The attorney general questioned whether the IDSA ignored crucial and relevant data about the seriousness and persistence of Lyme infections when it formulated its treatment guidelines, evidenced by quoting only two percent of the published literature about Lyme available at the time and ignoring treatment guidelines published two years earlier by the ILADS (Cameron et al., 2004). As a result of the settlement in April 2008, the IDSA agreed to voluntarily review its guidelines by convening an independent review panel. An ombudsman was selected to vet the members of the panel for potential conflicts of interest. After they reviewed the guidelines in their entirety with special attention to the recommendations for post-Lyme disease syndromes, the review panel concluded in 2008 that no changes to the guidelines were needed, insisting the 2006 guidelines were medically and scientifically justified on the basis of all available evidence (Lantos et al., 2010).
As the national debate about Lyme and related diseases continues, NatCapLyme remains committed to informing both the Lyme community and the public about the scientific and political developments surrounding tick-borne diseases so that affected patients may better advocate for their own healthcare.
Learn more:
Please note: NatCapLyme does not endorse or necessarily agree with the content found at the following sites.
Published Treatment Guidelines from the International Lyme and Associated Diseases Society (ILADS) published in the National Clearinghouse of Guidelines:
Other websites which may contain useful overviews of the crucial issues related to Lyme:
- Centers for Disease Control and Prevention (CDC)
http://www.cdc.gov/ncidod/dvbid/lyme/ - International Lyme and Associated Diseases Society (ILADS) Basic Information
http://www.ilads.org/lyme/about-lyme.php - Lyme and Tick-Borne Diseases Research Center, Columbia University
http://www.columbia-lyme.org/research/lymetbd_center.html - National Institutes of Health (NIH):
https://www.niaid.nih.gov/topics/lymedisease/Pages/lymeDisease.aspx - University of Rhode Island Tick Encounter Resource Center
http://www.tickencounter.org/
References
- Aberer E, Kersten A, Klade H, Poitschek C, Jurecka W. 1996 Dec. Heterogeneity of Borrelia burgdorferi in the skin. Am J Dermatopathol 18(6):571-9.
- Agosta F, Rocca MA, Benedetti B, Capra R, Cordioli C, Filippi M. 2006 Apr. MR imaging assessment of brain and cervical cord damage in patients with neuroborreliosis. AJNR Am J Neuroradiol 27(4):892-4.
- Alban PS, Johnson PW, Nelson DR. 2000 Jan. Serum-starvation-induced changes in protein synthesis and morphology of Borrelia burgdorferi. Microbiology 146( Pt 1):119-27.
- Barbour AG, Hayes SF. 1986. Biology of Borrelia species. Microbiol Rev 50(4):381-400.
- Barbour AG, Todd WJ, Stoenner HG. 1982. Action of penicillin on Borrelia hermsii. Antimicrob Agents Chemother 21(5):823-9.
- Benach JL. 1999 Dec 10. Functional heterogeneity in the antibodies produced to Borrelia burgdorferi. Wien Klin Wochenschr 111(22-23):985-9.
- Blanc F, GEBLY [Groupe d’Etude de la. Borréliose de Lyme]. 2007 Jul-Aug. [Neurologic and psychiatric manifestations of Lyme disease] Med Mal Infect 37(7-8):435-45.
- Breier F, Khanakah G, et al. 2001. Isolation and polymerase chain reaction typing of Borrelia afzelii from a skin lesion in a seronegative patient with generalized ulcerating bullous lichen sclerosus et atrophicus. Br J Dermatol 144(2):387-392.
- Brorson O, Brorson SH. 1998 Dec. A rapid method for generating cystic forms of Borrelia burgdorferi, and their reversal to mobile spirochetes. APMIS 106(12):1131-41.
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. 1982 Jun 18. Lyme disease-a tick-borne spirochetosis? Science 216(4552):1317-9.
- Cameron D, Gaito A, et al. ILADS Working Group. 2004. Evidence-based guidelines for the management of Lyme disease. Expert Rev Anti Infect Ther 2(1 Suppl):S1-13.
- Coulter P, Lema C, et al. 2005 Oct. Two-year evaluation of Borrelia burgdorferi culture and supplemental tests for definitive diagnosis of Lyme disease. J Clin Microbiol 43(10):5080-4.
- Dattwyler RJ, Volkman DJ, et al. 1988 Dec 1. Seronegative Lyme disease. Dissociation of specific T- and B-lymphocyte responses to Borrelia burgdorferi. New Engl J Med 319(22):1441-6.
© Copyright 2008 NatCapLyme. This article may be reproduced or linked with attribution and without modification.



